Background
Cytarabine resistance is a major reason for compromised treatment efficacy in acute myeloid leukemia (AML). This may be due to leukemic cells being at different stages in their cell cycle, with some at G0/G1, others in M phase, and only a limited number of cells in the S phase necessary for cytarabine susceptibility. Palbociclib, is a selective CKD4/6 inhibitor that arrests cancer cells in the G0/G1 stage. Preclinical studies have shown that the administration of palbociclib to AML cells in vitro for 48 hours can arrest 91% of the cells in the G0/G1 phase. When these cells proceed to S phase after the cessation of palbociclib, cytarabine-based (S phase dependent) chemotherapy has 30-50% enhanced cytotoxic activity.
We hypothesize that the sequential administration of palbociclib and CPX-351 can enhance its efficacy without added toxicity.
Methods
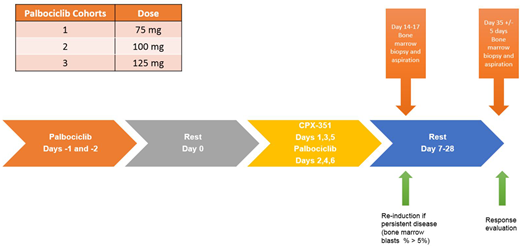
This is a phase 1 (dose escalation of palbociclib) /II single arm trial of the sequential administration of palbociclib and CPX-351. The trial consists of two components: a phase I, 3+3 design to evaluate the safety with dose escalation of palbociclib in combination with CPX-351 (for patients [pts] with relapsed/primary refractory [R/R] AML, or higher risk newly diagnosed AML); and a phase II to evaluate the overall response rate (ORR) of the combination in newly diagnosed AML pts. Palbociclib was given at dose level 1 (75 mg po) (Figure 1) on day -1 and -2, day 0 rest, followed by CPX-351 (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) on day 1, 3, and 5 along with palbociclib on day 2, 4, and 6 followed by a rest/monitoring period (days 7-35). Patients received 1-2 induction courses of the combination of palbociclib and CPX-351. Inclusion criteria: age 18-65, ECOG performance status (< 2), and adequate organ function. Pts with active infections or malignancies that prevented them from enrollment or with cardiac ejection fractions <45 were excluded. The primary endpoint for phase I was to evaluate the safety of the combination and for phase II to evaluate ORR defined by 2003 IWG criteria.
Results:
In this pre-specified analysis, 9 pts competed phase I (3 received palbociclib at 75 mg, 3-100 mg, and 3- 125 mg). The median age was 48 (range, 30-69), 44% females, and median WBC was 3.5 (range, 0.27-39.7). Cytogenetic analysis included: 2 pts with normal karyotype, 4 pts with complex karyotype, 1 pt with inversion 3, 1 pt with Trisomy 8, and 1 pt (no growth). Four pts had TP53 mutations. There were no reported deaths during induction. Common adverse events were similar to those was observed in pivotal CPX-351 trials. Common grade 3 / 4 side effects possibly related to therapy included: febrile neutropenia in 6 pts, elevated bilirubin in 1 pt, epistaxis in 1 pt, electrolyte abnormalities in 1 pt, and atrial fibrillation in 1 pt. All toxicities resolved during the study period. One pt also experienced grade 2 pericarditis/pericardial effusion (possibly related), which resolved. The ORR among the 8 evaluable pts (1 pt had persistent disease at day 14 and refused to receive re-induction and was taken off the study) was 75%: 5 pts (63%) achieved a CR and 1 pt (12%) had a morphological leukemia-free state.
A total 5 pts enrolled in the phase II trial. Three are evaluable for response, 1 withdrew from the study during induction therapy (pt choice), and one is still receiving therapy. Among the three evaluable pts one (unfavorable cytogenetics with a TP53 mutation) achieved CR, one (unfavorable cytogenetics achieved) CRp, and one (unfavorable cytogenetics) achieved CRi. No induction mortality has occurred to date. The phase II portion of the study is still ongoing.
Conclusions
The sequential combination of palbociclib and CPX-351 is highly effective in pts with R/R and newly diagnosed AML enrolled to this trial. The combination was well tolerated and no added toxicity was observed from palbociclib. The trial is ongoing and an updated enrollment of phase II pts will be presented at the meeting.
Nazha:MEI: Other: Data monitoring Committee; Jazz: Research Funding; Novartis: Speakers Bureau; Incyte: Speakers Bureau. Mukherjee:Aplastic Anemia and MDS International Foundation: Honoraria; Celgene/Acceleron: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Research Funding; Bristol Myers Squib: Honoraria; Partnership for Health Analytic Research, LLC (PHAR, LLC): Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; EUSA Pharma: Consultancy. Carraway:BMS: Consultancy, Other: Research support, Speakers Bureau; Stemline: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; ASTEX: Other: Independent Advisory Committe (IRC); Takeda: Other: Independent Advisory Committe (IRC); Abbvie: Other: Independent Advisory Committe (IRC); Novartis: Consultancy, Speakers Bureau. Gerds:Apexx Oncology: Consultancy; AstraZeneca/MedImmune: Consultancy; Incyte Corporation: Consultancy, Research Funding; Roche/Genentech: Research Funding; CTI Biopharma: Consultancy, Research Funding; Pfizer: Research Funding; Sierra Oncology: Research Funding; Celgene: Consultancy, Research Funding; Gilead Sciences: Research Funding; Imago Biosciences: Research Funding. Patel:Alexion: Other: educational speaker. Advani:Abbvie: Research Funding; Macrogenics: Research Funding; Glycomimetics: Consultancy, Other: Steering committee/ honoraria, Research Funding; Immunogen: Research Funding; Seattle Genetics: Other: Advisory board/ honoraria, Research Funding; Amgen: Consultancy, Other: steering committee/ honoraria, Research Funding; Kite: Other: Advisory board/ honoraria; Pfizer: Honoraria, Research Funding; Novartis: Consultancy, Other: advisory board; OBI: Research Funding; Takeda: Research Funding. Maciejewski:Alexion, BMS: Speakers Bureau; Novartis, Roche: Consultancy, Honoraria. Sekeres:Pfizer: Consultancy; BMS: Consultancy; Takeda/Millenium: Consultancy.
Palbociclib in AML CPX-351 in newly diagnosed AML
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal